
PRECISE PRO RX Carotid Stent System
The PRECISE PRO RX® Carotid Stent System's unique design is to enhance contourability and increase longitudinal stability and uniform scaffolding
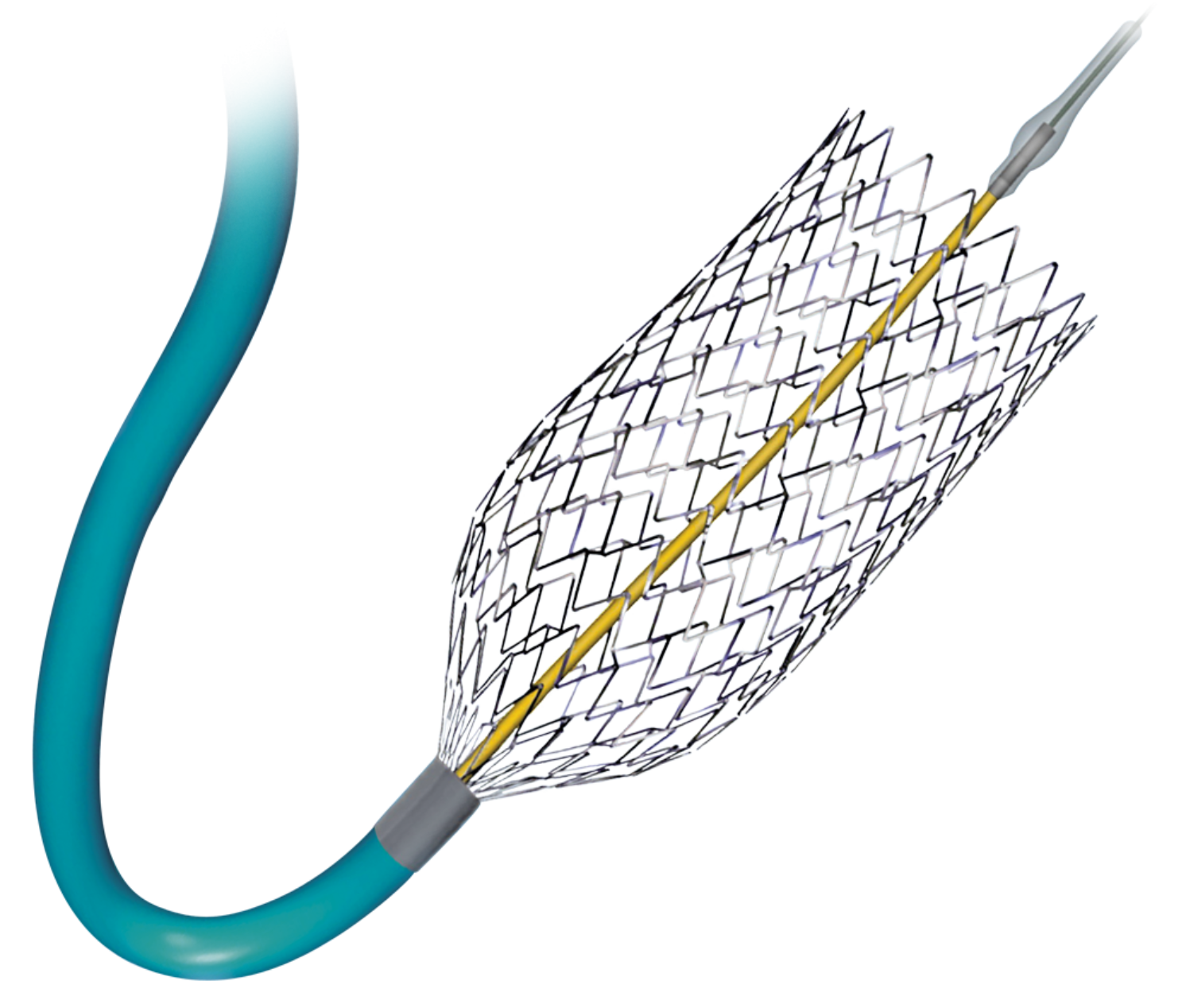
Product Description
The Cordis PRECISE PRO RX Stent offers:
- Excellent Contourability: Micromesh geometry and segmented design helps the stent conform to the artery wall; best in class wall apposition, autotapering design and excellent stent flexibility.
- Best in Class Radial Strength: Easily withstands pressure of the carotid artery, ability to resist recoil and maintain a gentle chronic outward force on vessel wall.
- Peak to Valley Design: Reduces kinking in bends, reduces the potential for "fish scaling" and provides best in class wall apposition.
- Accurate, Easy Placement: Minimal stent shortening, low profile design, Cordis Trumark Technology for accurate stent deployment. 5Fr compatible (5-8mm) 6Fr compatible (9-10mm).
IFU
Please refer to the Instructions for Use for complete information, including indications, precautions, warnings, and potential adverse events.
Customer Service and Ordering Information
In the United States, email us your question or order, or call us at 800.327.7714.

